|||
|
|
🗝 Login
🤖 Create Account
Main Menu
🚤 Model Boats
• Forum
• Build Blogs
• Media Gallery
• Boat Clubs & Lakes
• Events
• Boat Harbour
• How-To Articles
• Plans & Docs
• Useful Links
This Website
🔍 Search
📝 Guestbook
👨👩👧👦 Members (7,830)
📖 Quick Site Guide
📣 Support
👥 Membership
Hobby Supplies
🛍️ Online Shop
Not Registered
Go AD FREE & get your membership medal
BRONZE
Less Ads
SILVER
GOLD
Ad Free
Cancel
Anytime
Anytime
£2.50
£4.50
£6.50
Subscribe
Go AD FREE & get your membership medal
BRONZE
Less Ads
SILVER
GOLD
Ad Free
For A Whole Year!
£25
£45
£65
Donate
You Will Be Helping Towards:
Domain Fees
Security Certificates
iOS & Android App Fees
Website Hosting
Fast Servers
Data Backups
Upkeep & Maintenance
Administration Costs
Without your support the website wouldn't be what it is today.
Please consider donating towards these fees to help keep us afloat.
Read more
All donations are securely managed through PayPal.
Many thanks for your kind support
Without your support the website wouldn't be what it is today.
Please consider donating towards these fees to help keep us afloat.
Read more
All donations are securely managed through PayPal.
Many thanks for your kind support
Model Boats Website
Home
Forum
Build Blogs
Media Gallery
Boat Clubs & Lakes
Events
Boat Harbour
How-To Articles
Plans & Docs
Useful Links
Welcome to the Model Boats Website! A place for all model boaters!
Feel free to browse through the website, ask your questions, upload your photos or even start your own blog!
Feel free to browse through the website, ask your questions, upload your photos or even start your own blog!
Login To
Remove Ads
Remove Ads
Today's Question
What is the term for the triangular sail that sits ahead of the foremast of a sailing vessel?
ANSWER >>
>
Today's Wordle
7 LETTERS
>
Trending Now
Harbour
Re: (Tug Boat) Lulonga quarterwheeler
All very interesting! Thanks again Tom, this time for the links.
Lew
Florida ️, USA
🇺🇸 LewZ

5 minutes ago
Blog
Re: AMPHITRUCK at the beach.
THAT is pretty darn cool!
🇺🇸 DWBrinkman

3 hours ago
Wiki
Lipo battery - whether to switch
I am looking for help/guidance regarding switches in my motor circuit.
My questions are:
1. Should I put a switch on the battery supply ?
2. If so, what rating should it be ?
3. There is no switch to the receiver/ESC at present and I believe there should be one. Can anyone confirm this please.
Now, for reference, the circuit comprises the following:
A 5S 5000mAh 30C Lipo battery; 40A fuse; 90A marine ESC; 3 20A fuses; a 46x50 brushless motor; Flysky receiver. (as yet no switches)
Any guidance will be greatly appreciated.
Somethings word here. Can't seem to reply to your post but I can edit it. Lew
🇪🇸 Julio

6 hours ago
Gallery
Re: INGA IV
Really enjoyed the water line video. She’s a beauty !
🇬🇧 flaxbybuck

6 hours ago
Harbour
Re: Shrimp fishing boat Capt CJ
I added a dedicated water pump for extra cooling of the motor. It was running continuously when the boat was powered. However, at the scale speeds I run, it was not really needed. So I removed it. However it is easily plugged to any port on the receiver.
For now, I rely on the water scoop in back of the propeller to cool the motor and the ESC.
Isaac
🇺🇸 Isaac

7 hours ago
Harbour
Re: 5. Old Stern wheel steamer
Hi Mike,
as Alessandro wrote, the best is , when you can take a motor with an already integrated gearbox and proper DC engine . Mainly 12 DC , due to the usual battery voltage used in the model. It is necessary to choose a type of motor with an appropriately selected speed (150 - 200(300)rpm max.
By controlling the motor via ESC, you can simple choose the proper
low rotation speed.
🇨🇿 tomarack
8 hours ago
Forum
Question of the Day?
Slowly making a comeback...slowly!
🇺🇸 jumpugly

10 hours ago
Forum
Wordle of the Day?
Thanks for your encouragement Cashrc!
Made it in 3 without a „clue“!
Greetings Michel-C.
🇨🇭 Mike Stoney

11 hours ago
Forum
THE MOST ORIGINAL, STRANGE AND FAILURE SHIPS EVER BUILT IN THE WORLD.
The third ship in the list of strange, bizarre, failed ships is the battleship NOVGOROD.
Now the list has gone from four to six thanks to Cashrc who added: 1) an aircraft carrier which proved to be very useful but very original, perhaps unique (along with its twin) of its kind, and 2) a submarine which seems to have been a total failure.
As you can see from the photos and as mentioned in the first message, the feature that makes it unique and bizarre is the circular shape.
The intent was to create a platform as stable as possible for the large naval artillery of the time.
Vice Admiral Popoff's idea was realized in 1873.
The 2500 ton ship had a diameter of 36.9 meters and mounted 280 mm caliber guns.
Propulsion was guaranteed by six propellers at the stern, powered by as many steam turbines capable of delivering a power of 2400 hp (wikipedia reports a different, much higher value).
The six propellers should have made it more maneuverable than any other ship, this was the prediction but in reality it was almost impossible to maintain a course and it was very slow, just 6 knots of speed.
The ship was launched in 1873 in Nikolajev on the Black Sea, and was supposed to operate as a coast guard together with the ship Admiral Popoff, also circular but with a slightly smaller diameter, but with a greater displacement of 3647 tons.
As soon as she left the Dnieper River she became uncontrollable, she rotated out of control and caused illness to the crew who apparently were paralyzed by dizziness.
The circular platform was indeed much more stable than traditional ships as expected by its designer but the nautical qualities were terrible.
The biggest drawback of her hull shape was that it greatly reduced the rudder's ability to turn the ship by masking much of the water flow, so much so that it took up to 40-45 minutes to make a complete circle. The solution adopted was to use the engines for control and leave the rudder fixed, even if this reduced the speed of the ship.
Despite this obvious failure, Tsar Alexander II was favorably impressed by the stability of this ship and wanted the imperial yacht, the Livadia, to be built on the same principle but to look like a traditional ship.
Popoff then designed a hull that was not circular but elliptical with a length of 72 meters and a width of 47 meters. But this is another bizarre ship that perhaps deserves a separate mention.
Unlike the other ships on the list, this ship type operated for a period of time before being scrapped in 1911 and also participated in the Russo-Turkish War of 1877-78.
🇮🇹 AlessandroSPQR

13 hours ago
Blog
Re: Sun's out, Jonny's out
Awesome work! And the multi gang winch is very cool! Excellent Westeind!
🇺🇸 jumpugly

19 hours ago
Forum
mascotte...pilot cutter
Maybe this can help get you on the right sea lane Jacks.
Cheers, Doug
https://prints.rmg.co.uk/products/lines-rigging-plan-for-yacht-cariad-1904-ex-bristol-channel-pilot-cutter-m1135
https://www.google.com/search?client=firefox-b-d&q=The+mascotte+pilot+cutter+plan
🇩🇪 RNinMunich

19 hours ago
Forum
Relax for a few days.
Habe ich mir fast 'gedenkt' Wolle
🇩🇪 RNinMunich

1 day ago
Harbour
Re: Billings Smit Nederalnd
Looks like a great project!
🇺🇸 Peejay

1 day ago
Harbour
Re: Oppie
Stotty!
🇺🇸 jumpugly

1 day ago
Forum
34" Fairy Huntsman air scoops
Hi Olly.
If I send you the details for one of mine can you produce the CAD file at some point please. No rush as I have a way to go with my builds.
I've done all my scratch build drawings in Visio which can't produce the files for 3D printing. I've started to learn TurboCAD but haven't got very far!
Once I've got one CAD file I hope I can alter it for my other builds. They are all at a scale of 1:12 but the air inlets do vary in shape and size from model to model.
Chris
🇬🇧 ChrisF

1 day ago
Forum
eezebilt Cresta
Hi Jacko, What motor and batteries are you running in your Cresta?
Do you do propellor swaps when you are out trying your boat out when first running?
Was she on full throttle at all or was there lot's more to go.
Was your Cresta driver just a little nervous when he was out on his first maiden trial run.
I am just wondering if she can get up on plane when running being a speed boat.
🇬🇧 BOATSHED
1 day ago
|
New Member
United Kingdom
 JollyRoger
JollyRoger9 minutes ago
New Member
United Kingdom
 PhilipP
PhilipP60 minutes ago
New Member
United Kingdom
 michaeld1
michaeld11 hour ago
New Member
Switzerland
 maran49
maran4911 hours ago
New Member
United Kingdom
 jacks
jacks22 hours ago
New Member
United Kingdom
 georgeC2
georgeC22 days ago
New Member
United Kingdom
 JohnJ1
JohnJ13 days ago
New Member
United Kingdom
 Geoffreym1
Geoffreym13 days ago
New Member
Spain
 JosLuisB
JosLuisB3 days ago
New Promotion
Midshipman
 EdW
EdW4 days ago
New Member
Argentina
 MatiasO
MatiasO4 days ago
New Member
United Kingdom
 BridgetH
BridgetH4 days ago
New Member
United Kingdom
 Jim1
Jim14 days ago
New Member
United States
 DanO
DanO5 days ago
New Promotion
Able Seaman
 Steves-s
Steves-s5 days ago
New Promotion
Captain
 Brightwork
Brightwork5 days ago
Birthday This Week
Turns 67
 Stephen T
Stephen T6 days ago
Birthday This Week
Turns 79
 Robert 87
Robert 876 days ago
New Member
United Kingdom
 DougW
DougW6 days ago
New Member
Germany
 UlfF
UlfF6 days ago
New Promotion
Leading Seaman
 impartit
impartit6 days ago
New Member
Norway
 ToreL
ToreL6 days ago
New Member
United Kingdom
 GregG1
GregG16 days ago
New Member
Turkey
 hakank
hakank6 days ago
New Member
Brazil
 OlimpioA
OlimpioA7 days ago
New Promotion
Leading Seaman
 whittonm
whittonm7 days ago
New Member
Australia
 JimP
JimP8 days ago
New Promotion
Captain
 flaxbybuck
flaxbybuck8 days ago
New Member
Hong Kong
 tufub
tufub8 days ago
New Promotion
Commodore
 AlessandroSPQR
AlessandroSPQR8 days ago
New Member
Australia
 GregH
GregH9 days ago
New Member
Egypt
 MohamedG
MohamedG9 days ago
Birthday This Week
Turns 78
 flaxbybuck
flaxbybuck9 days ago
New Member
United Kingdom
 bennob
bennob9 days ago
Birthday This Week
Turns 75
 gallego
gallego10 days ago
New Promotion
Commodore
 Doogle
Doogle10 days ago
New Member
United Kingdom
 Bob Upndown
Bob Upndown10 days ago
New Promotion
Lieutenant Commander
 Wolle
Wolle10 days ago
New Member
United Kingdom
 Cyril
Cyril10 days ago
Account Updated
Updated Signature
 Pete 46
Pete 4611 days ago
Account Updated
Updated 'About Me'
 Pete 46
Pete 4611 days ago
New Member
United Kingdom
 Pete 46
Pete 4611 days ago
New Member
 Fadiyassine
Fadiyassine11 days ago
New Member
United Kingdom
 MarkT
MarkT11 days ago
New Member
Australia
 Lsgc
Lsgc11 days ago
New Member
United Kingdom
 PeterF2
PeterF211 days ago
New Member
United Kingdom
 MichaelS2
MichaelS211 days ago
New Member
United Kingdom
 geraldl
geraldl11 days ago
See More
Forum Topics
Question of the Day?
Slowly making a comeback...slowly!
10 hours ago by 🇺🇸 jumpugly ( Lieutenant Commander)
Lieutenant Commander)
 Lieutenant Commander)
Lieutenant Commander)
Website Related
2364 Posts
6060 Likes
6060 Likes
Started
1 year ago
by fireboat
1 year ago
by fireboat
Latest
10 hours ago
by jumpugly
10 hours ago
by jumpugly
Wordle of the Day?
👍👍👍
Thanks for your encouragement Cashrc!
Made it in 3 without a „clue“! 😁
Greetings Michel-C.
11 hours ago by 🇨🇭 Mike Stoney ( Commander)
Commander)
 Commander)
Commander)
Website Related
1275 Posts
2635 Likes
2635 Likes
Started
10 months ago
by fireboat
10 months ago
by fireboat
Latest
11 hours ago
by Mike Stoney
11 hours ago
by Mike Stoney
THE MOST ORIGINAL, STRANGE AND FAILURE SHIPS EVER BUILT IN THE WORLD.
The third ship in the list of strange, bizarre, failed ships is the battleship NOVGOROD.
Now the list has gone from four to six thanks to Cashrc who added: 1) an aircraft carrier which proved to be very useful but very original, perhaps unique (along with its twin) of its kind, and 2) a submarine which seems to have been a total failure.
As you can see from the photos and as mentioned in the first message, the feature that makes it unique and bizarre is the circular shape.
The intent was to create a platform as stable as possible for the large naval artillery of the time.
Vice Admiral Popoff's idea was realized in 1873.
The 2500 ton ship had a diameter of 36.9 meters and mounted 280 mm caliber guns.
Propulsion was guaranteed by six propellers at the stern, powered by as many steam turbines capable of delivering a power of 2400 hp (wikipedia reports a different, much higher value).
The six propellers should have made it more maneuverable than any other ship, this was the prediction but in reality it was almost impossible to maintain a course and it was very slow, just 6 knots of speed.
The ship was launched in 1873 in Nikolajev on the Black Sea, and was supposed to operate as a coast guard together with the ship Admiral Popoff, also circular but with a slightly smaller diameter, but with a greater displacement of 3647 tons.
As soon as she left the Dnieper River she became uncontrollable, she rotated out of control and caused illness to the crew who apparently were paralyzed by dizziness.
The circular platform was indeed much more stable than traditional ships as expected by its designer but the nautical qualities were terrible.
The biggest drawback of her hull shape was that it greatly reduced the rudder's ability to turn the ship by masking much of the water flow, so much so that it took up to 40-45 minutes to make a complete circle. The solution adopted was to use the engines for control and leave the rudder fixed, even if this reduced the speed of the ship.
Despite this obvious failure, Tsar Alexander II was favorably impressed by the stability of this ship and wanted the imperial yacht, the Livadia, to be built on the same principle but to look like a traditional ship.
Popoff then designed a hull that was not circular but elliptical with a length of 72 meters and a width of 47 meters. But this is another bizarre ship that perhaps deserves a separate mention.
Unlike the other ships on the list, this ship type operated for a period of time before being scrapped in 1911 and also participated in the Russo-Turkish War of 1877-78.
13 hours ago by 🇮🇹 AlessandroSPQR ( Commodore)
Commodore)
 Commodore)
Commodore)
General Resources
15 Posts
61 Likes
61 Likes
Started
3 days ago
by AlessandroSPQR
3 days ago
by AlessandroSPQR
Latest
13 hours ago
by AlessandroSPQR
13 hours ago
by AlessandroSPQR
mascotte...pilot cutter
Maybe this can help get you on the right sea lane Jacks.
Cheers, Doug😎
https://prints.rmg.co.uk/products/lines-rigging-plan-for-yacht-cariad-1904-ex-bristol-channel-pilot-cutter-m1135
https://www.google.com/search?client=firefox-b-d&q=The+mascotte+pilot+cutter+plan
19 hours ago by 🇩🇪 RNinMunich ( Fleet Admiral)
Fleet Admiral)
 Fleet Admiral)
Fleet Admiral)
Building Related
2 Posts
3 Likes
3 Likes
Started
22 hours ago
by jacks
22 hours ago
by jacks
Latest
19 hours ago
by RNinMunich
19 hours ago
by RNinMunich
Relax for a few days.
Habe ich mir fast 'gedenkt' Wolle😁
😎
1 day ago by 🇩🇪 RNinMunich ( Fleet Admiral)
Fleet Admiral)
 Fleet Admiral)
Fleet Admiral)
Non-Hobby Chat
48 Posts
251 Likes
251 Likes
Started
12 days ago
by Wolle
12 days ago
by Wolle
Latest
1 day ago
by RNinMunich
1 day ago
by RNinMunich
|
|
Login To
Remove Ads
Remove Ads
Build Blogs
25 Posts
16 Followers
171 Likes
THE AMPHITRUCK
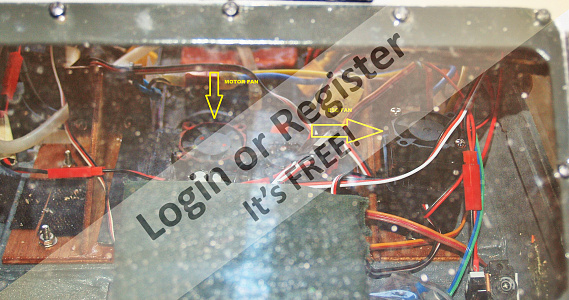
Decided to do something other than a boat this time, but still with a nautical purpose,- a 6 wheel twin diff twin prop swimming truck. Been done before, but I thought I'd have a go at one of my own design. Drew up some side and end templates and made a start, Purchased all the mechanical bits, (diffs, front axle, prop shafts, props, ESC, wheels,) over a few months from uncle ALI and added them to stuff I had in my collection.
Using cheap 3mm packing ply, I made the floor base ( to suit the diff and front axle widths) and strengthened it with 10x10 ally angle. Next,-cut out the sides and front and back panels. The floor was scored at the front and back to allow it to be bent up to the sides for gluing. Holes were cut in the floor to allow the diff heads to protrude through.
I had to modify the front axle to suit the width of the diffs by adding in an ally angle center section. Also had a play with setting the diffs and drive-shafts up with universals. Drive motor is a 380 1500 RPM @ 12v geared reduction drive, which is the one I first used in my Jeep tow wagon for my Hartley, (replaced it with a 1000 RPM version for more torque) and uses a 3s LiPo and Quicrun 40A ESC.
🇳🇿 jbkiwi

9 hours ago


















3 Posts
8 Followers
44 Likes
Aeronaut Jonny
Hi all, I started a new build during Christmas and I'm just about ready to start painting the haul so I figured its a good time to get the build log going.
I've had this kit for about a year now, purchased from Bauer in Germany along with with the recommended equipment including their Schottel Drive system. The plan is to have the two Schottel drives, a bow thruster, one working Anchor (possibly two in the future of off the same winch), a sound system, two working radars, working spot light with pivot in pitch and yaw, always on nav lights and interior lights, RC working main winch and manually operated bow and side winches, tow separate working fire monitors able to rotate individually, possibly a smoke generator and final an auto bilge system This comes to a total of 18 RC, unfortunately I only have 16 available so we will have to see what ends up in the ship.
So far I have the drives and bow thruster all fit and ready for assembly. I then built up the frame work and made the holes for the fire pumps and bilge. then had to build the lower deck house so I could fit the deck in the right place on the ship. Once the deck was dry fit I cut the scuppers then glued the deck down. I then set to work making the deck splash proof by installing and extending the kits coaming. I also add a drop floor under the aft hatch that I'm hoping will allow me to keep the water that gets on the deck from leaking down into the haul. Now I've got the Bulwark stanchions and handrails just about complete. I also started work on some of the deck items as I need the locations to work out some of the modifications I'm working through.
I hope to start painting the haul soon, I'm going to try brush painting this ship as I was able to find some nice paints at an Art supply store and I don't have much access to a place to spray paint in the winter. I do think I will spray on the primer first and hope it helps absorbed some of the brush marks.
🇨🇦 Westwind

1 day ago

















56 Posts
16 Followers
344 Likes
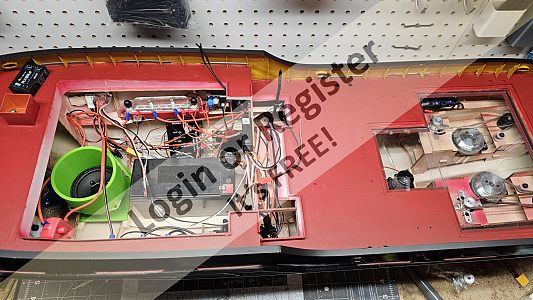
Constellation
Made the framed glass portion of the skylight. They're hinged so I can get a finger inside to flip the power switch on or off.
They're made from clear plastic from some packaging, basswood, and brass wire.
🇺🇸 Jerry Todd

5 days ago






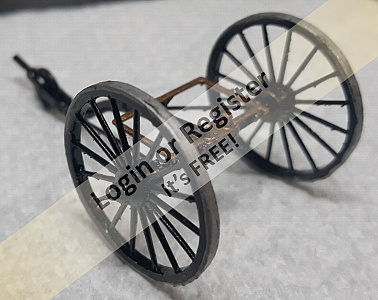








9 Posts
19 Followers
117 Likes
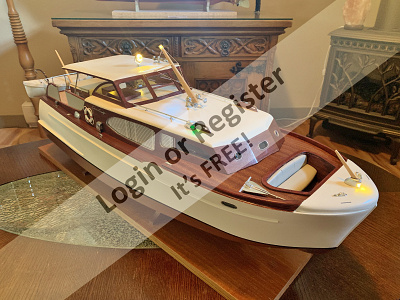
1949 40' Chris Craft Challenger
Hello all.... I have started a new project!! progress so far... she will be 32 " LOA and twin screw.
🇨🇦 Brightwork

5 days ago


















29 Posts
19 Followers
370 Likes
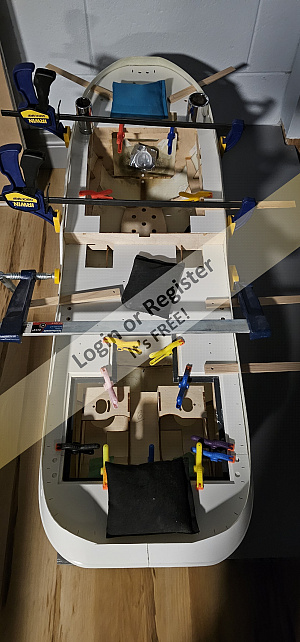
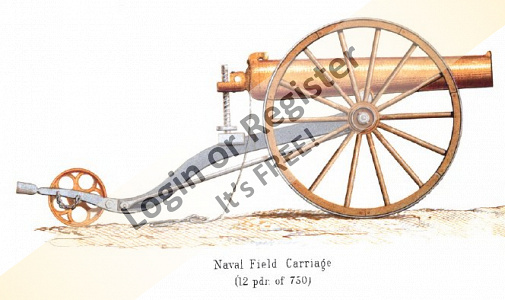
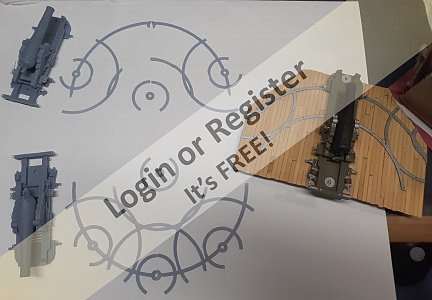
Ship of the desert ? new project
Started the latest project. Spotted these nice 105mm rubber tyred wheels in the big hardware store, and thought they deserved having something to be attached to,- hence the new project.
Scratch building using my 'cut it and see' method Started with a 20x20mm ally angle frame (main V from one piece angle cut and bent with bent and bolted rear cross bar) Rear axle is 10x1mm ally tube, with stub axles (from 10mm bright steel) turned down to fit inside and turned down for a 6mm securing Nyloc nut, and a rear shoulder for the wheels.
Stub axles and tube are drilled through 3mm and secured with 3mm machine screws.
Front axle is 6mm rod from an old sponge mop, and is bent and angled for a bit of reverse castor. 50x3mm ally strip added to the front of the frame for reinforcing and to mount 'stuff' to. The front steering head bush is 10mm bright steel drilled 6mm to be attached to?
Tune in next time for another thrilling instalment
JB
🇳🇿 jbkiwi

5 days ago



















1 Post
5 Followers
3 Likes
To switch or not to switch ?
I wrongly entered my last post in the 'how to' section where, it seems, you are unable to respond. So here is my query for which I am seeking your guidance.
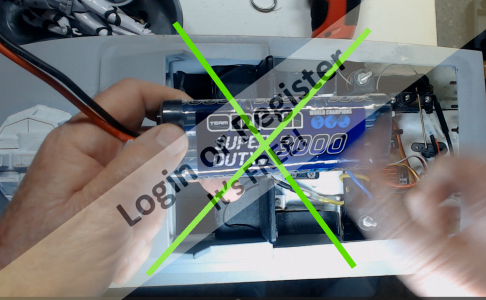
I have a motor circuit that comprises : 5S 5000mAh 30C Lipo; 40A fuse; 90A marine ESC; 3 x 20A fuses; 46 x 50 870KV brushless motor. There are no switches in the circuit yet.
1.Should I have a switch serving the battery ?
2. If yes, what rating ?
3. Should I have a switch between the ESC and the receiver ?
Any advice or guidance gratefully received.
🇬🇧 flaxbybuck

6 days ago

37 Posts
21 Followers
249 Likes
A new build
I will, most likely, have to rename this Blog, at some point.
In the mean time...please do not expect much for a while as she is in her very early stages.
First a disclosure: This hull was built for me by another gentleman. It was delivered in a not quite completed state. Therefore I have spent the last two month working on it to get to it's present state.
So.. what is it? She is one of four ships in a class that was later enlarged to around eight maybe ten members of the class. Her main armament was eight 5"/38 caliber twin turrets. She and her class namesake sister were both lost during the Naval Battle of Guadalcanal. This ship is most noted due to the loss of an entire generation of one family, five brothers.
This should be enough for identification.
She is being built in my preferred scale, 1/48th or 1/4"=1'.
This ship was used for numerous camouflage scheme experiments and, sadly, there is little definitive documentation to pinpoint her second to last and last schemes carried. That said, my plan, subject to change without notice or reason, is to present her as she appeared following her New York Navy Yard overhaul, or perhaps as she may have appeared after repainting in Placentia Bay NewFoundland, 1942.
She measures out to 11' 3" and her power plant is to be two 24 volt Buehler motors.
Pictures of her and the build to follow.
Wish me luck please, she will not fit in my van....
🇺🇸 ToraDog

6 days ago
















3 Posts
11 Followers
38 Likes
Aeronaut Graf Spee
I bought this kit about 10 years ago from a German model shop. It is definitely old-school, with great detailed plans, detailed stock list, but no instructions beyond a couple of paragraphs. I have not built a model in 20 years but was very active in my youth 50 years ago. I find that my skills have deteriorated and this will not be a great build, but it is enjoyable solving the problems and figuring out how to build this thing.
It has a plastic hull, a very nice plastic fitting set, but the rest of the kit is wood, with many pieces which have to be cut from printed sheets. Luckily, I have access to a wood shop with jigsaw and sanders.
I have fitted out the hull, added the motors, and started the superstructure. Stay tuned for further progress, I hope.
🇨🇦 whittonm

7 days ago





1 Post
1 Follower
7 Likes
52' Motor Lifeboat Victory
One of my nine-year projects is coming to an end. I am sad to see it completed in some ways, overjoyed in others. Many projects have been begun and shelved over those nine years; some are in the trash now.
What is known is the 213' WMEC-168 Yocona is hit or miss whether my attempts to waterproof a static hull will be successful. So on hold or a side project.
The Gunboat Philadelphia is on-going at work between shifts.
PBY that doesn't Fly I will place on hold, pending possible actual flight. (That will put three aircraft inline to be completed.)
The battleship North Carolina....Big. Not so complex as physically large undertaking.
SO, begin at the beginning as I tell my students. I pondered purchasing the laser-cut kit from Canada, but it is just frames, deck, and pilothouse. Yes, it is 1" to one foot scale, and would match my 44' MLB perfectly, but $286 plus shipping? For me too much, I will build at 1/24th scale for now. Small enough to store, big enough to detail and outfit with running gear.
My 44 MLB, is a leaky, but I am installing an automatic bilge pump. I get so much joy out of running it, and I will have the tri-fecta of MLB's 36', 44', and 52'. The 213 should be complete around the time I finish, so four CG projects in a years time, plus the Philly is a quick build. So, it is a year, year and a half plan.
This will be a slow one.
Non Boat picture is project that is almost complete.
Kevin
🇺🇸 KevinH

8 days ago


1 Post
6 Followers
11 Likes
Kathryn - a Thames Bawley - 10
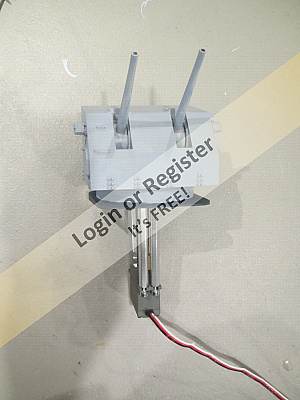
About the mainsail, topsail and large staysail.
The gaff is lifted by the uphaul (Pic 1) with the halyard being tied off on a mast cleat (Pic 2) Note this is not how the real thing would be done, but is my method that works on a model.
There are a number of cleats attached to the foot of the mast. These are glued and attached using long screw eyes ( see earlier blog)
Pic 3 shows the gaff 'spreader' (my term) the halyard passing through various pulleys before travelling down the mast to a cleat (Pic4) More about pulleys in the next Blog.
The following photos show the principle sails and how they are attached. I have made a number of other sails not shown here, including a smaller main sail and topsail, a smaller staysail, a jib and a flying jib. These can be set to suit different wind strengths.
The mainsail (pic 5 ) is an old cotton sheet stained with tea. I have sewn reinforcement patches into the places that will come under stress or tension, and bias binding onto all edges. Metal eyes or hooks are then sewn into the clew (Pic 6), the tack (Pic 7), the throat (Pics 8 and 9), and the peak (Pic 10 ). Pic 11 just shows how the bias binding is used to finish the sail edges.
Attachment points are shown in the next few pictures. Pic 12 the tack eye, Pic 13 the throat eye, Pic 14 the peak uphaul and Pic 15 the clew outhaul. Pic 16 shows the main sheet emerging onto the deck, passing through an eye on the travel horse and heading for the main boom.
The topsail is shown in Pic 17, already stained with Colron dye, corners reinforced and bias binding sewn on. The following five pictures show the topsail foot, clew and peak in detail.
Pic 23 is the large staysail, with the tack, clew and head shown in the final pictures.
None of the methods used for attaching these sails is authentic. My aim is to be able to sail the boat and enjoy seeing it on the water. I therefore need to be able to attach or detach the sails quickly at the pondside. I use a variety of attachments, principally eyes and hooks. I try to make these as neat as possible, and not detract from the appearance.
We often talk about 'passing the ten foot test', meaning if it looks OK when ten feet away, then it is OK. However, viewing these photos I can see just how poorly I have finished off sewing in the sail corners. There are too many ragged edges and untidy hand sewing. In future I must remind myself of this and do better. (Sounds like my school reports - 'must do better !')
🇬🇧 flaxbybuck

8 days ago



















5 Posts
4 Followers
41 Likes
Star Wars Patrol Boat (1/6 scale)
This is a model I built several years ago. Someone gave me an old wood hull which I turned into an experimental boat, and wound up being a Star Wars Storm Trooper patrol boat. I generally don't do many "funny" boats and try to keep everything to scale. I believe this is a good example of making a comical boat to scale (no stuffed animals, etc.).
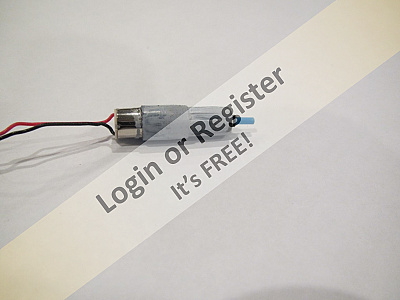
It never performed well as it was small and the six "C" cell battery pack weighted the boat down giving very little freeboard (about 3/8 inch) and a slow speed.
I decided to replace the 3000MAH 7.2V Nimh battery with a 2S Li-ion 3500MAH &.4V battery. Last Sunday I took it to the SSMBC meet and it went very well but after 50 feet it died. (It was the motor.) So that is now replace and it will be over two weeks to the next meet.
So, in the mean time I took some photos of the fix-up and "innards". In particular you can see the adapter I made to fit the 2S battery where the hobby battery sat. It is 3D printed. I placed the file on "Thingiverse" (link below) in case anyone else is interested.
I plan to get some videos at the next meet.
Lew
Florida , USA
https://www.thingiverse.com/search?q=7.2V+Hobby+Battery+adapter+for+2S+Li-ion+&page=1
🇺🇸 LewZ

9 days ago












7 Posts
16 Followers
81 Likes
Chris Craft Cobra
New project is well underway. 1955 18' CC Cobra in 1/8 scale. Framework, chines, sheers and transom all together. I am starting the planking today. The last picture shows the boat I am building. I don't think the hardtop is factory but I really like the looks of it. So I will attempt a scratch built hard top. I will update soon. Thanks, RR
P.S. We got my Mom a new condo and this is looking out her back patio I Love it!!!
🇺🇸 River Rat

9 days ago

















|
|
Media Gallery
INGA IV
9 hours ago by philcaretaker
Lake Union Dreamboat
3 days ago by Brightwork
1958/9 50/55' Chris Craft Constellation
4 days ago by Brightwork
Filming "Dinghy Dan"
16 days ago by philcaretaker
"Dinghy Dan"
16 days ago by philcaretaker
"Dan, Dan the Dinghy Man"
19 days ago by philcaretaker
"Dinghy Dan"
19 days ago by philcaretaker
RNLI Severn Class
20 days ago by Rudy-M
Onboard a Focus 2 RC yacht
20 days ago by Rudy-M
Andy sails Topaz with df65's
20 days ago by MartinH2
RC Shark vs Orca?
22 days ago by Rudy-M
Topaz 60" J class yacht
25 days ago by MartinH2
|
|
Login To
Remove Ads
Remove Ads
Boat Clubs & Lakes
Recent Updates In Places

|
Model Boating Association of South Africa
9 days ago by 🇿🇦 Rudy-M (
 Warrant Officer) Warrant Officer) |

|
BUXTON MODEL BOAT CLUB
19 days ago by 🇬🇧 philcaretaker (
 Commodore) Commodore) |

|
Rawdon Model Boat Club
19 days ago by 🇬🇧 MikeC3 (
 Petty Officer 2nd Class) Petty Officer 2nd Class) |

|
Sonstraal Dam
29 days ago by 🇿🇦 Rudy-M (
 Warrant Officer) Warrant Officer) |

|
Cape Town Scale Model Boat Club
29 days ago by 🇧🇪 hermank (
 Captain) Captain) |
|
|
Upcoming Events

|
Apr
14 2024
|
1 Day Only!
|
Bring and Buy 2024
Ended 6 days ago
|

|
Apr
14 2024
|
1 Day Only!
|
Opening of the electro driven boats
Ended 6 days ago
|

|
May
5 2024
|
1 Day Only!
|
Edina Model Yacht Club - Spring Breakfast
Starts 15 days time
|

|
Jun
8 2024
|
1 Day Only!
|
RAWDON MBC OPEN DAY
Starts 2 months time
|

|
Jun
9 2024
|
1 Day Only!
|
Edina Model Yacht Club - Parade of Boats at Centennial Lakes Ponds
Starts 2 months time
|
|
|
Boat Harbour
7 Photos
9 Likes
Oppie
Way back when, there used to be a company producing grp hulls, they were based in Holyhead(?) or nearby, the business was sold, and I discovered they had some pieces for the Oppie, I bought them, constructed the model, sailed it, was impressed, so much so I embarked on the construction of the other exact 1:4 scale models.
🇫🇷 stotty1111

1 day ago
0 Attributes
3 Comments
3 Photos
15 Likes
Graupner Commodore
I acquired this Commodore and made some modifications so she is now a Chris Craft Roamer 37'
🇨🇦 Brightwork

5 days ago
4 Attributes
10 Comments
2 Photos
18 Likes
Billings Smit Nederalnd
I aquired this for free and did some restoration on her. I will probably give her to my brother.
🇨🇦 Brightwork

5 days ago
4 Attributes
9 Comments
5 Photos
12 Likes
1949 40' Chris Craft Challenger
This is based on the Dumas model plans. Model was produced by dumas as a kit in the 50's. I semi scratch built her from the plans.
🇨🇦 Brightwork

5 days ago
8 Attributes
5 Comments
9 Photos
11 Likes
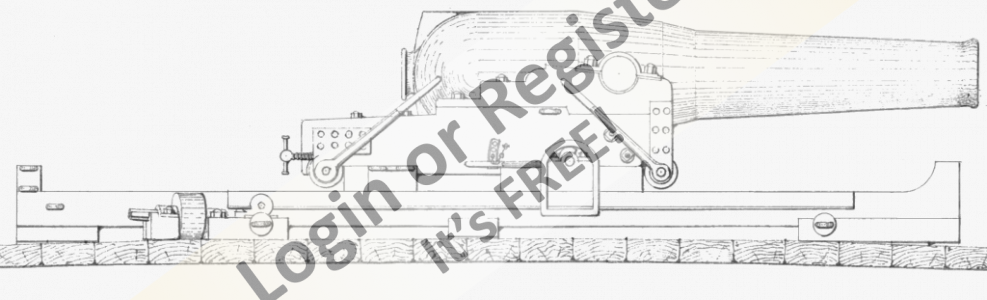
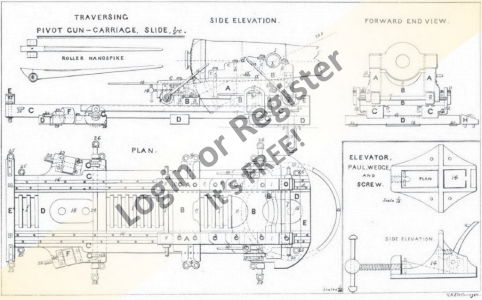
Steam Power
Not strictly marine based, but the Stuart D10 is destined for a 1:12 Customs Launch, Active, built by Cox & Co Falmouth 1912, and stationed at Gravesend until the '30's.
Kit of castings, machined and built by myself along with the horizontal engine.
The others have been bought at knock down prices over the years!
They all need sprucing up!
The boiler was bought several years ago from an Australian outfit, at the time it seemed incredibly cheap, but was probably caused by the exchange rates between the Australian dollar and the pound!
It came complete with lagging, planking and other bits and pieces, cannot be exact as I cannot find the box with the pieces in, cannot remember if it was complete with burner!
I now have the thermal blocks (ex night storage heater) to construct a hearth to continue with the installation etc.
Photos of 'Active' to follow it is stored at the club house!
🇫🇷 stotty1111

11 days ago
0 Attributes
2 Comments
9 Photos
22 Likes
Oystercatcher
Here we have Oystercatcher, another product of the noughties.
The full size boat is designed for inland waters, weekend cruising, it can be used as a floating tent using the spars and sails.
Designed by Conrad Natzio of East Anglia, it is designed for garage construction, using 6 sheets of marine ply or even water resistant ply.
I met Conrad at an exhibition at Alexandra Palace, had a chat with him was given plans of several of his designs, chose to build Oystercatcher, kept in touch and was invited to a weekend expo at Beale Park, adjacent to the Thames, he used my model on his stand, a very nice man!
The model is an exact scale of 1:4, I enjoyed the build, but the control was not an easy installation, the boat being so open.
Went on to sail her at an exhibition held in Swansea at the Maritime Museum, they had a pond there so was able to try sailing, it worked well, but I felt I needed to add a keel to bring a little more stability, but house moves etc got in the way.
Makes a nice static model!
I will endeavour to get more photos when the wind is calmer!
🇫🇷 stotty1111

14 days ago
0 Attributes
6 Comments
2 Photos
11 Likes
Mini Submarine
Another toy, courtesy of Amazon 7 or 8 years ago.
I bought the beast for about £12 / 15€. It was on offer!
I use it at model exhibitions where there is a pool.
The sub is about 15cm/6" in length, complete with basic r/c system.
It will sail forwards and reverse, turns to the right and left courtesy of water jets, and by use of 2 buttons on the top corners will submerge and resurface. It has rechargeable batteries inside, these are recharged by use of a fly lead from the tx!
The main users are children and sometimes adults who want to have a go!
It generates lots of interest, so much so that sometimes children join the club and go on to build small freelance fishing boats - the majority of models are fishing and military boats, we have the Brest naval base close by and several fishing ports.
🇫🇷 stotty1111

16 days ago
0 Attributes
6 Comments
13 Photos
24 Likes
1:4 scale Clinker Dinghy
Built around 2000 whilst I worked as a Technician in the Design Technology section of a local senior school, built at home, but with pieces produced in the woodworking shop, having access to a plethora of workshop equipment!
Sailed somewhat unsuccessfully back then, I had problems finding sail material for its Bermudan rig, my idea was to convert to gaff style rig, never got around to it, however now with more choice sail cloth wise I might have another go, however my sailmaker died some time ago!
The major problem sailing was the launching and retrieving, the weight was around 8 - 9 kgs(18lbs), and with age that is probably more of a problem, although here in France there is more attention given to those sort of problems!
The model won a gold award at a model show in Concarneau in 2003, after which I was invited to join my local club in Chateaulin -- the best move I made!
🇫🇷 stotty1111

17 days ago
0 Attributes
6 Comments
1 Photo
19 Likes
Two Mast Gaff Rigged Hampton Boat
My first scale model boat. Plank on Frame. Double planked with Mahogany outer. Sealed with Clear Epoxy resin. Fitted with lights, nav lights, anchor winch and a bilge pump. (Slightly modified wheelhouse)
A link to the plans on Pinterest is below. I added one more rib to the mid section and moved the fore mast to the aft.
https://za.pinterest.com/pin/pin-su-sailboat-bucket-list--489133209526451555/
🇿🇦 Rudy-M

22 days ago
3 Attributes
9 Comments
|
|
How-To Articles
Lipo battery - whether to switch
Revised 6 hours ago
By flaxbybuck
LED – SERIES AND PARALLEL CIRCUITS – FLASHING CIRCUITS - Compendium of information
Revised 14 days ago
By AlessandroSPQR
Transmitter Camera Mount
Revised 1 month ago
By cjanik001
NAVAL PROPELLERS. Compendium of information.
Revised 1 month ago
By AlessandroSPQR
Lipo Battery Reference charts
Revised 2 months ago
By CB90
K.M.Y.C.A. Monthly magazine
Revised 3 months ago
By hermank
CALCULATION OF THE IMMERSED VOLUME (SIMPLE MATHEMATICAL/GEOMETRIC METHOD) OF YOUR MODEL
Revised 4 months ago
By AlessandroSPQR
Basic Model Boat Operations
Revised 4 months ago
By LewZ
Fiberglass boat hull
Revised 5 months ago
By northark
Bending Small Diameter Copper Tubing
Revised 6 months ago
By LewZ
|
|
Login To
Remove Ads
Remove Ads
🛍️ Basket
Main menu transported here on mobile
Login
Create New Account
Trending Topics
Members Online
Harbour
Re: (Tug Boat) Lulonga quarterwheeler
LewZ
5 minutes ago
Blog
Re: AMPHITRUCK at the beach.
DWBrinkman
3 hours ago
Wiki
Lipo battery - whether to switch
Julio
6 hours ago
Gallery
Re: INGA IV
flaxbybuck
6 hours ago
Harbour
Re: Shrimp fishing boat Capt CJ
Isaac
7 hours ago
Harbour
Re: 5. Old Stern wheel steamer
tomarack
8 hours ago
Forum
Question of the Day?
jumpugly
10 hours ago
Forum
Wordle of the Day?
Mike Stoney
11 hours ago
Forum
THE MOST ORIGINAL, STRANGE AND FAILURE SHIPS EVER BUILT IN THE WORLD.
AlessandroSPQR
13 hours ago
Blog
Re: Sun's out, Jonny's out
jumpugly
19 hours ago
Forum
mascotte...pilot cutter
RNinMunich
19 hours ago
Forum
Relax for a few days.
RNinMunich
1 day ago
Harbour
Re: Billings Smit Nederalnd
Peejay
1 day ago
Harbour
Re: Oppie
jumpugly
1 day ago
Forum
34" Fairy Huntsman air scoops
ChrisF
1 day ago
Forum
eezebilt Cresta
BOATSHED
1 day ago
Blog
Re: sails
RossM
2 days ago
Forum
My sailing waters in Stade.
hermank
2 days ago
Gallery
Re: Lake Union Dreamboat
jumpugly
3 days ago
Gallery
Re: 1958/9 50/55' Chris Craft Constellation
Brightwork
3 days ago
Harbour
Re: Graupner Commodore
Rudy-M
3 days ago
Forum
Smoke spaghetti
Fred
3 days ago
Forum
Today at our pond, IngaIV and tug Portbalne
AlessandroSPQR
3 days ago
Forum
Yorkshire Belle
Rowen
3 days ago
Forum
Deans Marine
Razor1955
3 days ago
Blog
Re: CGINGA1V
jbkiwi
4 days ago
Forum
Modello RC scala 1/60, di piroscafo armato a goletta, liberamente ispirato alle cannoniere classe US
AlessandroSPQR
4 days ago
Harbour
Re: 1949 40' Chris Craft Challenger
Frankiesays1953
4 days ago
JollyRoger
Recruit
0 Points
4 seconds ago

flaxbybuck
Captain
3,073 Points
13 seconds ago

mdsrecycles
Recruit
0 Points
17 seconds ago

AlessandroSPQR
Commodore
4,297 Points
26 seconds ago

RossM
Sub-Lieutenant
1,464 Points
38 seconds ago
DWBrinkman
Lieutenant Commander
2,429 Points
38 seconds ago

jbkiwi
Fleet Admiral
19,344 Points
39 seconds ago

BOATSHED
Lieutenant
1,894 Points
49 seconds ago

LewZ
Captain
3,696 Points
4 minutes ago

neilw
Lieutenant
1,923 Points
6 minutes ago

BarryW
Recruit
0 Points
10 minutes ago

DavidE
Recruit
0 Points
21 minutes ago

Rowen
Captain
3,481 Points
22 minutes ago

PhilipP
Recruit
0 Points
39 minutes ago

Niels
Recruit
0 Points
1 hour ago

Wolle
Lieutenant Commander
2,241 Points
1 hour ago

michaeld1
Recruit
0 Points
1 hour ago

PhilH
Warrant Officer
623 Points
1 hour ago

Peejay
Midshipman
1,051 Points
1 hour ago

mturpin013
Admiral
8,351 Points
1 hour ago

luckyduck
Midshipman
969 Points
2 hours ago

MikeN12
Recruit
0 Points
2 hours ago

MauriceL
Able Seaman
27 Points
2 hours ago

NickD
Warrant Officer
843 Points
2 hours ago

Cyril
Recruit
0 Points
3 hours ago

Schifty1
Recruit
13 Points
3 hours ago

ChrisF
Lieutenant Commander
2,165 Points
3 hours ago

tomarack
Midshipman
990 Points
3 hours ago

jalvarro
Recruit
0 Points
3 hours ago

River Rat
Captain
3,184 Points
3 hours ago

boatmam
Recruit
0 Points
4 hours ago

Rudy-M
Warrant Officer
766 Points
4 hours ago

Biggles1
Recruit
2 Points
4 hours ago

Olly999
Able Seaman
40 Points
4 hours ago

surfacesub
Recruit
18 Points
4 hours ago

Eboatt
Recruit
2 Points
4 hours ago

Alain
Recruit
17 Points
4 hours ago

tkiddle
Recruit
0 Points
4 hours ago

PeterH6
Recruit
0 Points
5 hours ago

hermank
Captain
3,284 Points
5 hours ago

jonbliss152
Leading Seaman
70 Points
5 hours ago

AustinG
Chief Petty Officer 2nd Class
410 Points
5 hours ago

Steam-mad
Able Seaman
34 Points
5 hours ago

stotty1111
Chief Petty Officer 1st Class
566 Points
5 hours ago

Julio
Petty Officer 1st Class
228 Points
6 hours ago

JOHN
Midshipman
924 Points
6 hours ago

DaveHJ
Recruit
0 Points
6 hours ago

Albert90
Petty Officer 1st Class
266 Points
6 hours ago

Westwind
Chief Petty Officer 1st Class
496 Points
6 hours ago

Willem
Master Seaman
127 Points
6 hours ago

Login To
Remove Ads
Remove Ads

🏠
Home
Home
📰
Trending
Trending
💬
Forum
Forum
🗝
Login / Join
Login / Join
|
Cookies are used for ads personalisation.
By using this website you agree to our use of cookies. More Info |
Main Menu
🚤 Model Boats
• Forum
• Build Blogs
• Media Gallery
• Boat Clubs & Lakes
• Events
• Boat Harbour
• How-To Articles
• Useful Links
This Website
🔍 Search
📝 Guestbook
👨👩👧👦 Members (7,830)
📣 Support
Hobby Supplies
🛍️ Online Shop
Login
🗝 Login
🗝 Create New Account
▼
Media Gallery
X
1 of 4
► |
◄ |
Media Viewer
^
_
X
Share
X
Flag Inappropriate Post
X
| Select Reason | |
| Sexual content Includes graphic sexual activity, nudity, and other sexual content. | |
| Violent or repulsive content Violent or graphic content, or content posted to shock viewers. | |
| Hateful or abusive content Content that promotes hatred against protected groups, abuses vulnerable individuals, or engages in cyberbullying. | |
| Harmful dangerous acts Content that includes acts that may result in physical harm. | |
| Child abuse Content that includes sexual, predatory or abusive communications towards minors. | |
| Promotes terrorism Content intended to recruit for terrorist organisations, incite violence, glorify terrorist attacks, or otherwise promote acts of terrorism. | |
| Spam or misleading Content that is massively posted or otherwise misleading in nature. | |
| Infringes my rights Privacy, copyright and other legal complaints. | |
Basket Updated
X
Loading...
Loading
Loading Uploader...









